Please enter your details to get this resource.
Email before download
Semantic Analytics: Integrated approach for pharmacovigilance teams to achieve awareness
[Whitepaper]

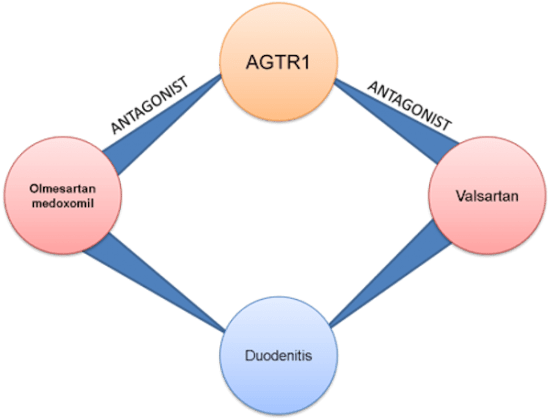
Regulatory bodies expect pharmaceutical companies to maintain an up-to-date awareness of the safety implications of not only their own drugs but also those from the same drug class and with the same target that are marketed by competitors. Comprehensive, systematic monitoring is required in order to detect, validate and act upon new adverse events as early as possible.
This places significant demands on Pharmacovigilance teams, who are challenged to maintain safety and compliance amid increasingly stringent, globally diverse regulations. The legacy approach, involving manually scanning biomedical sources, is prohibitively time consuming, has a high risk of missing safety signals and is no longer a viable option.
SciBite provides a resource-effective solution to the challenges faced by Pharmacovigilance teams by unlocking the potential of unstructured biomedical content. With semantic analytics, pharmaceutical companies can monitor a wide range of heterogeneous and cross-disciplinary sources and reach timely, well-informed decisions, resulting in safer treatments for patients.
To find out more, download the full whitepaper.
Related articles
-
Drug repurposing, rare diseases and semantic analytics
In this blog we cover how to look potentially reduce the cost of and speed up the repurposing pipeline.
Read -
GSK Japan selects semantic platform to enhance pharmacovigilance capabilities
SciBite today announced that GSK Japan, one of Japan’s leading research-based pharmaceutical and healthcare companies, has selected SciBite’s Semantic Platform to enhance pharmacovigilance capabilities and deliver on its commitment to improve the quality of human life.
Read
How could the SciBite semantic platform help you?
Get in touch with us to find out how we can transform your data
© Copyright © 2024 Elsevier Ltd., its licensors, and contributors. All rights are reserved, including those for text and data mining, AI training, and similar technologies.